Karl Fischer Moisture Titration
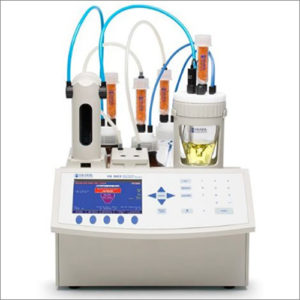
Karl Fischer titration is an accurate method to determine trace amounts of water in a sample using uses coulometric or volumetric titration.
- Description
| Testing Method | Karl Fischer Moisture Titration |
| Description | Karl Fischer titration is an accurate titration method in chemical analysis that uses coulometric or volumetric titration to determine trace amounts of water in a sample. It was invented in 1935 by the German chemist Karl Fischer. Today, the titration is done with an automated Karl Fischer titrator.
The elementary reaction responsible for water quantification in the Karl Fischer titration is oxidation of sulfur dioxide with iodine:
H2O + SO2 + I2 → SO3 + 2HI
This elementary reaction consumes exactly one molar equivalent of water vs. iodine. Iodine is added to the solution until it is present in excess, marking the end point of the titration, which can be detected by potentiometry. The reaction is run in an alcohol solution containing a base, which consume the sulfur trioxide and hydroiodic acid produced.
There are two methods used to perform the Karl Fischer titration test. One is known as Volumetric Karl Fischer. The other method is known as Coulometric Karl Fischer. The advantage of the Coulometric Karl Fischer method is the capability to accurately measure small amounts of moisture. Sensitivity of these instruments is as low as 0.1 microgram (µg) of water. This method is normally used for moisture content below 1% or for samples where the moisture is less than 200 micrograms. The volumetric method is ideal when working with samples containing higher levels of moisture (generally over 1% or 2%) but also when samples may contain ketones and or aldehydes. |
| More Information | Wikipedia: Karl Fischer Titration |