Mass Spectrometry (Q-TOF-MS)
Mass spectrometry (Q-TOF-MS) is an analytical technique that ionizes chemical species and sorts the ions based on their mass to charge ratio.
- Description
|
Testing Method |
Mass Spectrometry (Q-TOF-MS) |
|
Description |
Mass spectrometry (MS) is an analytical technique that ionizes chemical species and sorts the ions based on their mass to charge ratio. In simpler terms, a mass spectrum measures the masses within a sample. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.
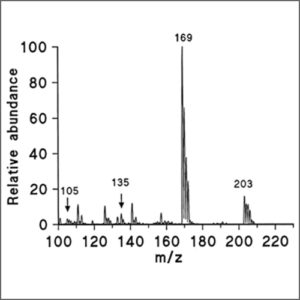
A mass spectrum is a plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical structures of molecules, such as peptides and other chemical compounds.
In a typical MS procedure, a sample, which may be solid, liquid, or gas, is ionized, for example by bombarding it with electrons. This may cause some of the sample’s molecules to break into charged fragments. These ions are then separated according to their mass-to-charge ratio, typically by accelerating them and subjecting them to an electric or magnetic field: ions of the same mass-to-charge ratio will undergo the same amount of deflection. The ions are detected by a mechanism capable of detecting charged particles, such as an electron multiplier. Results are displayed as spectra of the relative abundance of detected ions as a function of the mass-to-charge ratio. The atoms or molecules in the sample can be identified by correlating known masses to the identified masses or through a characteristic fragmentation pattern.
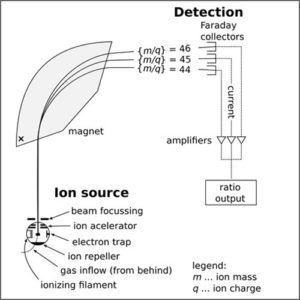
Ion Source
The ion source is the part of the mass spectrometer that ionizes the material under analysis (the analyte). The ions are then transported by magnetic or electric fields to the mass analyzer. Techniques for ionization have been key to determining what types of samples can be analyzed by mass spectrometry.
Mass Analyzer
Mass analyzers separate the ions according to their mass-to-charge ratio. The time-of-flight (TOF) analyzer uses an electric field to accelerate the ions through the same potential, and then measures the time they take to reach the detector. If the particles all have the same charge, the kinetic energies will be identical, and their velocities will depend only on their masses. Ions with a lower mass will reach the detector first.
Time-of-flight mass spectrometry (TOF-MS) is a method of mass spectrometry in which an ion’s mass-to-charge ratio is determined via a time measurement. Ions are accelerated by an electric field of known strength. This acceleration results in an ion having the same kinetic energy as any other ion that has the same charge. The velocity of the ion depends on the mass-to-charge ratio (heavier ions of the same charge reach lower speeds, although ions with higher charge will also increase in velocity). The time that it subsequently takes for the ion to reach a detector at a known distance is measured. This time will depend on the velocity of the ion, and therefore is a measure of its mass-to-charge ratio. From this ratio and known experimental parameters, one can identify the ion.
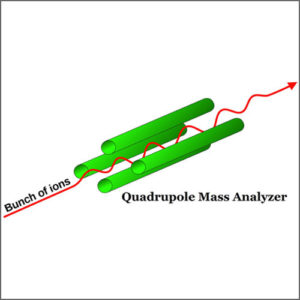
Quadrupole mass analyzer uses oscillating electrical fields to selectively stabilize or destabilize the paths of ions passing through a radio frequency (RF) quadrupole field created between 4 parallel rods. Only the ions in a certain range of mass/charge ratio are passed through the system at any time, but changes to the potentials on the rods allow a wide range of m/z values to be swept rapidly, either continuously or in a succession of discrete hops. A quadrupole mass analyzer acts as a mass-selective filter and is closely related to the quadrupole ion trap, particularly the linear quadrupole ion trap except that it is designed to pass the untrapped ions rather than collect the trapped ones, and is for that reason referred to as a transmission quadrupole. A magnetically enhanced quadrupole mass analyzer includes the addition of a magnetic field, either applied axially or transversely. This novel type of instrument leads to an additional performance enhancement in terms of resolution and/or sensitivity depending upon the magnitude and orientation of the applied magnetic field. A common variation of the transmission quadrupole is the triple quadrupole mass spectrometer. The “triple quad” has three consecutive quadrupole stages, the first acting as a mass filter to transmit a particular incoming ion to the second quadrupole, a collision chamber, wherein that ion can be broken into fragments. The third quadrupole also acts as a mass filter, to transmit a particular fragment ion to the detector. If a quadrupole is made to rapidly and repetitively cycle through a range of mass filter settings, full spectra can be reported. Likewise, a triple quad can be made to perform various scan types characteristic of tandem mass spectrometry.
Quadrupole time-of-flight mass spectrometer (Q-TOF-MS) refers to a mass selecting quadrupole and collision quadrupole with time-of-flight device as the second mass selection stage. Q-TOF-MS is used for the mass spectrometry of peptides and other large biological polymers. |
|
More Information |
Wikipedia: Mass Spectrometry ; Time-Of-Flight Mass Spectrometry ; Quadrupole Mass Analyzer |