Hot
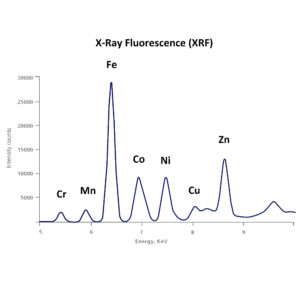
X-Ray Fluorescence (XRF)
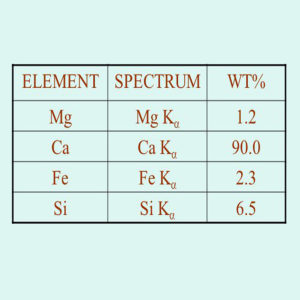
X-ray fluorescence (XRF) is an analytical technique that can be used to determine the chemical composition of a wide variety of sample types.
- Description
| Testing Method | X-Ray Fluorescence (XRF) |
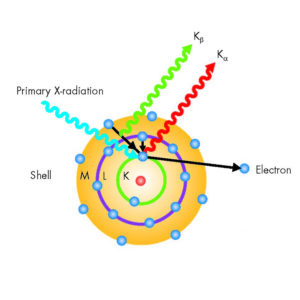
| Description | X-ray fluorescence (XRF) is the emission of characteristic “secondary” (or fluorescent) X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings and murals. In principle, the lightest element that can be analysed is beryllium (Z = 4), but due to instrumental limitations and low X-ray yields for the light elements, it is often difficult to quantify elements lighter than sodium (Z = 11), unless background corrections and comprehensive inter-element corrections are made.
The technology used for the separation (dispersion), identification and intensity measurement of a sample’s X-ray fluorescence spectrum gives rise to two main types of spectrometer: wavelength dispersive (WDXRF) and energy dispersive (EDXRF) systems. EDXRF spectrometers are different from WDXRF spectrometers in that they are smaller, simpler in design and have fewer engineered parts, however the accuracy and resolution of EDXRF spectrometers are lower than for WDXRF. |
| More Information | Wikipedia: X-Ray Fluorescence |