Circular Dichroism Spectroscopy (CD)
Circular dichroism spectroscopy (CD) uses circularly polarized light to investigate structural aspects of optically active chiral media.
- Description
| Testing Method | Circular Dichroism Spectroscopy (CD) |
| Description | Circular Dichroism (CD) is an absorption spectroscopy method based on the differential absorption of left and right circularly polarized light. Optically active chiral molecules will preferentially absorb one direction of the circularly polarized light.
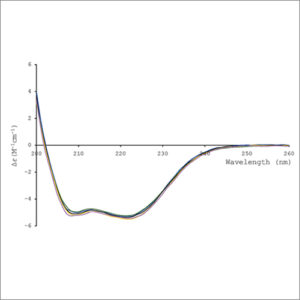
In general, CD phenomenon will be exhibited in absorption bands of any optically active molecule. As a consequence, circular dichroism is exhibited by biological molecules, because of their dextrorotary and levorotary components. Even more important is that a secondary structure will also impart a distinct CD to its respective molecules. Therefore, the alpha helix of proteins and the double helix of nucleic acids have CD spectral signatures representative of their structures. The capacity of CD to give a representative structural signature makes it a powerful tool in modern biochemistry with applications that can be found in virtually every field of study. The far-UV (ultraviolet) CD spectrum of proteins can reveal important characteristics of their secondary structure. The near-UV CD spectrum (>250 nm) of proteins provides information on the tertiary structure. Visible CD spectroscopy is a very powerful technique to study metal–protein interactions and can resolve individual d–d electronic transitions as separate bands.
CD spectroscopy is a quick method that does not require large amounts of proteins or extensive data processing. Thus CD can be used to survey a large number of solvent conditions, varying temperature, pH, salinity, and the presence of various cofactors.
|
| More Information | Wikipedia: Circular Dichroism |