Ion Chromatography (IC)
Ion chromatography is a modified version of HPLC with a capacity for precise and highly sensitive detection of inorganic ions in a complex matrix.
- Description
| Testing Method | Ion Chromatography |
| Description | Ion Chromatography is a method for separating ions based upon their interactions with resin (stationary phase) and the eluent (mobile phase). These phases differ between an anion column, which attracts anions, and a cation column, which attracts cations. Each column can only measure conductivity of the specific type of ion that it attracts. Ions will move through the columns of the ion chromatographer at different speeds depending on their affinity for the specific resin, and they will separate from each other based upon differences in ion charge and size. As the eluent passes through the column, ions with a weaker affinity for the resin will move through the column faster and be eluted first, while ions with a stronger affinity for the column will move through the column more slowly.
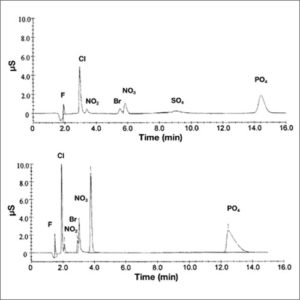
Upon exiting the column, the ions are measured by an electrical conductivity detector. This detector produces a chromatogram which plots conductivity vs. time. Each ion produces a peak on this graph, the height of which is dependent on the relative ion concentration in the injected solution. These measurements can then be used to determine concentrations of analytes in an unknown sample. To combat possible interference caused by the ions in the mobile phase, a suppressor may be used to remove the unwanted electrolyte prior to the conductivity measurement. As the solution passes through the suppressor, ions in the eluent are replaced with a nonionic species. Alternatively, if the eluent is sufficiently dilute or has a low conductivity, the use of a suppressor is not necessary.
Since 1975 ion chromatography has been widely used in many branches of industry such as environment, electricity, sanitation, energy, agriculture, food and beverage, pharmaceutical, chemical, petrochemical and many other fields. The main beneficial advantages are reliability, very good accuracy and precision, high selectivity, high speed, high separation efficiency, and low cost of consumables. Ion chromatographs are able to measure concentrations of major anions, such as fluoride, chloride, nitrate, nitrite, and sulfate, as well as major cations such as lithium, sodium, ammonium, potassium, calcium, and magnesium in the parts-per-billion (ppb) range. Concentrations of organic acids can also be measured through ion chromatography. |
| More Information | Wikipedia: Ion Chromatography |